Critical Elements and Metals
Out of the 100+ elements on the Periodic Table of Elements, and many more in the tomes used by mineralogists, chemists and geophysicists, there are some that are considered to be strategic for issues related to national and economical security. These so-called critical elements and metals; many of which are heavily employed in emerging technologies – as well as making preexisting technologies much more efficient – have unique properties that aren’t replicated by their cousin elements. Theoretical technologies of the past can be realized because of these special properties, and are now sought after by nations powerful enough to be in competition.
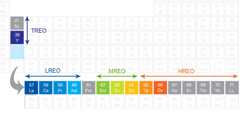
Subsets of the substances that fall under the designation of being critical elements or metals are the technology metals, such as Tellurium, Scandium, Gallium, Indium, Selenium, Germanium, Vanadium, Cadmium, Rhenium, Molybdenum and more. Some of the rare earth metals are even more instrumental in present day technologies, and only expected to be more in the future: neodymium, praseodymium, europium, samarium, cobalt, dysprosium and others. Consider some of their specific uses in order to appreciate their designation as critical elements:
- Tellurium is favored for its use in many alloys; usually for the purposes of enhancing the machinability (it makes steel, copper and other metals easier to mold without breakage) of the main substance. This critical energy element finds extensive application in semiconductors – the basis of just about everything modern technology has to offer – and solar panels, which are expected to grow drastically in use as we move towards a greener economy.
- Scandium is critical because of its sheer rarity. Generally, the atoms of the metal are prevalent mostly in stars; the silver-white rare earth metal finds excellent use in light bulbs, because of its ability to emit high-intensity light that closely resembles that of sunlight. Most importantly, employing scandium as an alloying agent with the light metal aluminum results in much stronger aircraft, which has obvious benefits, especially since it actually makes them lighter in weight, as well as cheaper in the case of mass production. The rarity of scandium makes it one of the more critical and strategic elements out of the collection here.
- Gallium is deeply silver metallic color, and maintains a liquid form at room temperature, despite having the characteristic properties of a metal with free electrons roaming the surface. Because of this, it finds use in high-temperature thermometers, which have a wide variety of advanced applications. Gallium also forms spectacular mirrors when painted on a glass surface, which can be useful for lasers and other things. However; by far its most important application is in the field of semiconductors, where its alloy with arsenic forms gallium arsenide, used for photovoltaic purposes and solid-state electronics. And we have yet to touch on gallium’s biomedical and astrophysical applications.
With these considerations in mind, the delineation of criticality for any mineral or element might be a fluid classification, because of the strategic need for Research and Development into finding viable substitutes, or presently hidden sources – or new liaisons with countries harboring large amounts. Brazil has nearly the entire planet’s store of recoverable niobium, for example, which is a lustrous grey metal that is instrumental in superconducting materials, as well as for alloying metals with the kind of incredible strength and resiliency needed in jet engine parts. The nuclear industry also makes use of niobium, and superconducting magnets are a near-term technological innovation that will revolutionize everything from the field of medicine, to general electronics.